Products lineup
iLiNP1.0, 2.0 (innovative Lipid Nanoparticles Production device)
– Low installation cost.
– Pump sold separately.
LiNAS series (Lipid Nanoparticles Assembling System)
– Low running cost. (low-cost microfluidic chip.)
– Pump sold separately for LiNAS-mini only.
(LiNAS-S will be available after 2024.)
LiMAP series (Lipid Nanoparticles Manufacturing Platform)
– Model for development and commercial production of pharmaceutical nanoparticle formulations.
(Sales scheduled for 2024 or later.)
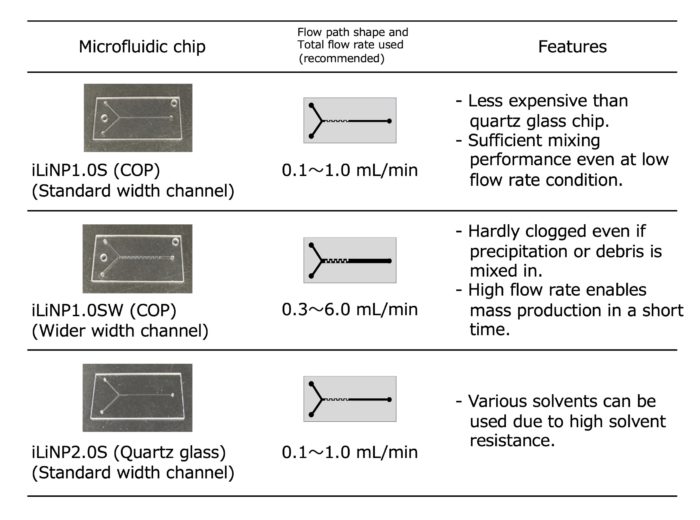
Microfluidic device iLiNP1.0, 2.0
・This model consists of a microfluidic chip and tubing connection components (accessories).
・By combining with a commercially available pump, an experimental environment for nanoparticle formulation can be easily constructed.

<Microfluidic chips (as of October 2023)>
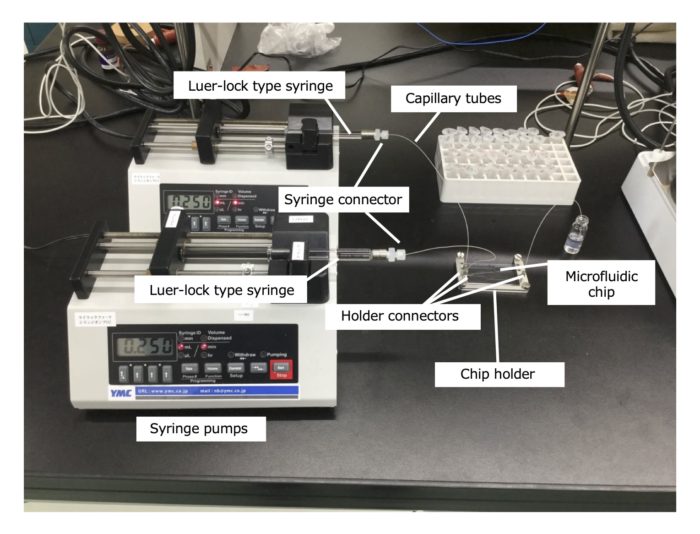
<Accessories for iLiNP series>
1. Chip holder S (ACC-CHO02S)
2. Holder connector and ferrule for OD0.5mm capillary tube (ACC-HCF01)
3. OD0.5mm PEEK capillary tube (ACC-PEK01)
4. Accessories set (1+2+3) for iLiNP series (ACC-SET01S)
5. Syringe connector and ferrule for OD0.5mm capillary tube (ACC-SCF01)
Accessories for 1/16″ OD tubing are also available. Please contact us for details.
<Configuration (an example)>
<Microfluidic chip selection guide>
– For low-cost small-scale prototyping ・・・ COP-iLiNP1.0S
– Want to use organic solvents other than alcohols ・・・ QUA-iLiNP2.0S
– Want to use at a high flow rate ・・・ COP-iLiNP1.0SW
LiNAS series
・This model can be used with low-cost PDMS microfluidic chips.
・The lineup includes three types: mini, which is used by connecting a commercially available pump, and LiNAS-S and LiNAS-M platforms, which are equipped with two dedicated syringe pumps.
1. Microfluidic chips for LiNAS series (LM series)
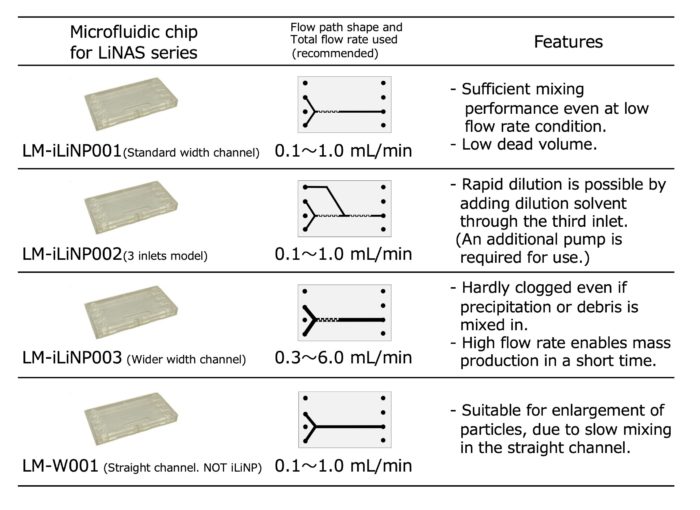
<Assembly of the microfluidic device>

If the microfluidic chip is damaged, only the chip can be replaced.
2. Systems for LM series microfluidic chip
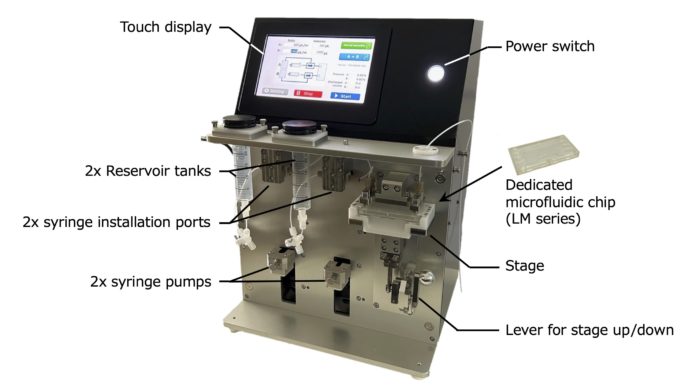
2-1. LiNAS-M
New version LM-003 is launched in October 2023.
・Multifunctional model equipped with two syringe pumps that allow free flow rate setting.
・Anyone can easily and reproducibly manufacture lipid nanoparticles and polymer nanoparticles of various sizes.
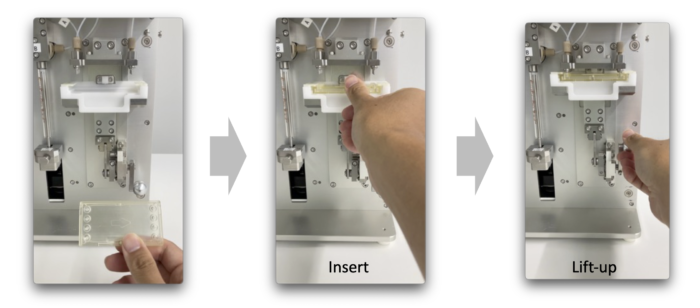
<Features #1>
Easy installation of the microfluidic device to the device by simply lifting up the microfluidic device.
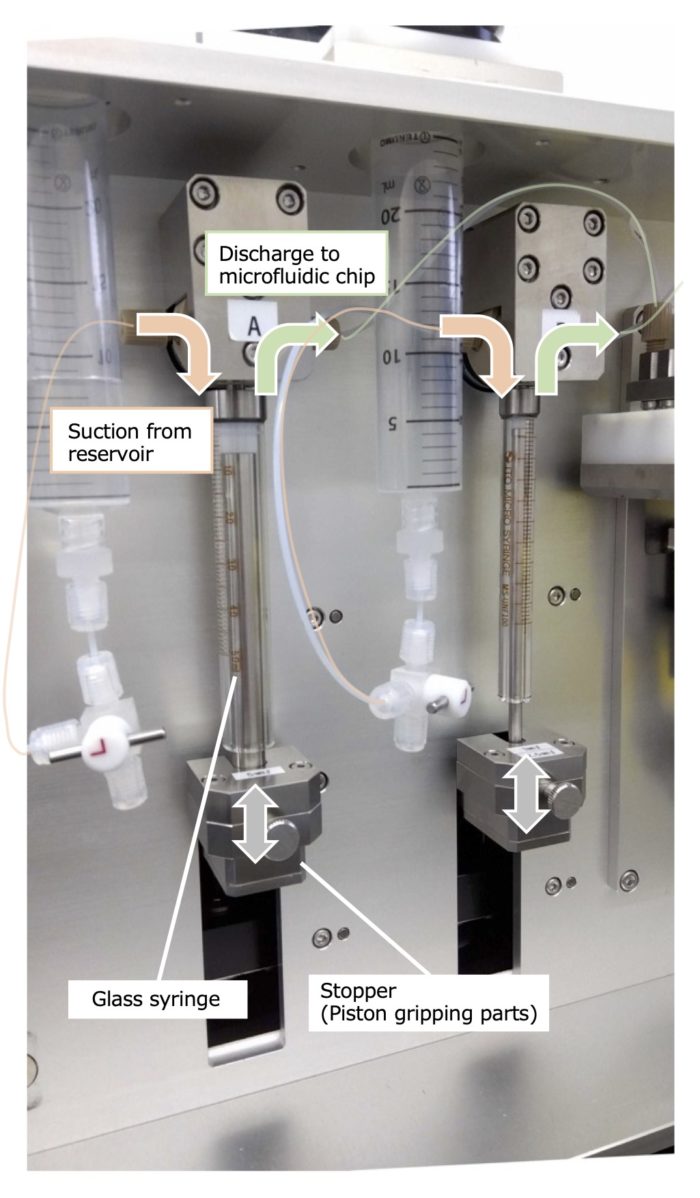
<Features #2>
Glass syringes and disposable syringes can be used separately depending on the purpose and application.
The use of the glass syringes is recommended if you want to try several pumping conditions using the same raw material solution or if you want to prepare a large quantity of nanoparticles by repeating pumping in combination with using reservoir.
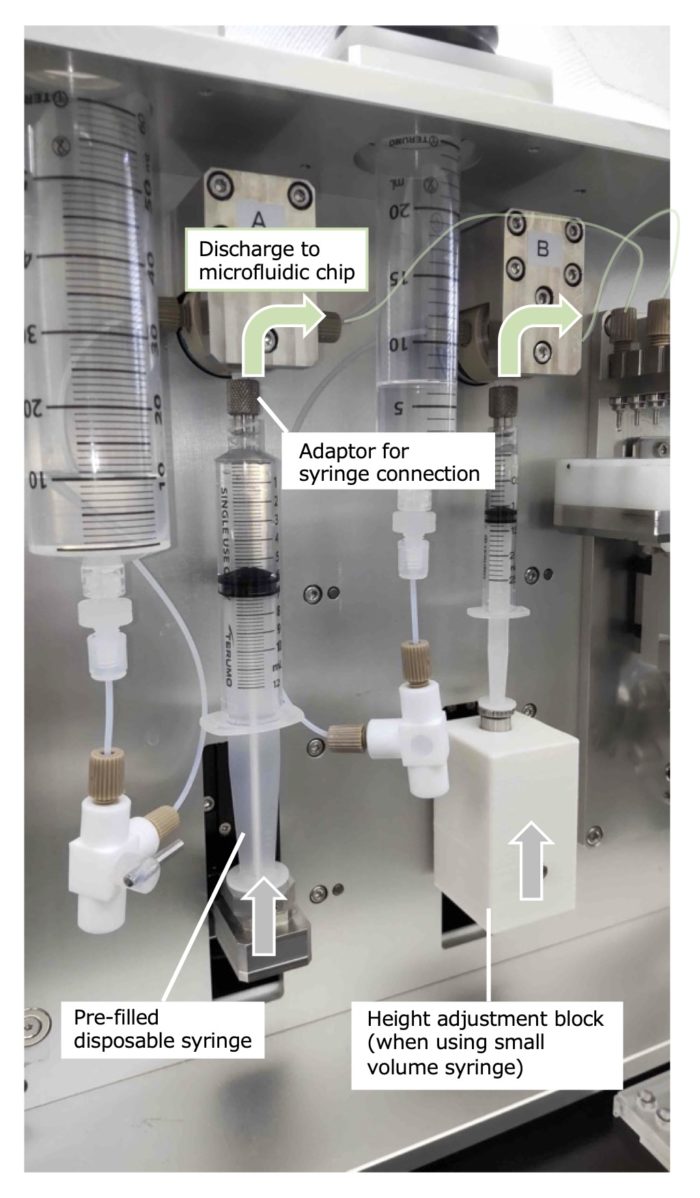
The use of the disposable syringes is recommended if you want to prepare prototype samples using different types of raw material solutions or if want to avoid the hassle of syringe cleaning.
<Features (others)>
・Easy to operate even for beginners.
・High reproducibility of nanoparticle production is achieved by using a high-precision pump.
・High flexibility of piping allows customers to easily customize the pumping route.
・Unlimited use of the dedicated microfluidic chip.
・Easy to control particle size by using iLiNP type microfluidic chip (patented technology).
・Flexibility to change the shape of the flow channel.
2-2. LiNAS-S (Sales scheduled for 2024 or later.)
・Simple model with focused functions.
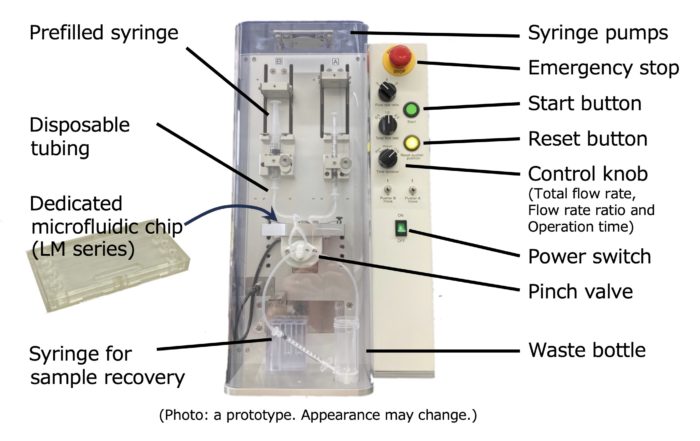
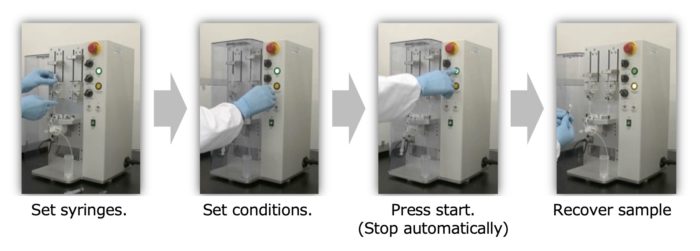
<Features #1>
After setting the pumping conditions, simply press the start button. Pumping is automatically terminated.
<Features (others)>
・Total flow rate, flow rate ratio and operation time can be set from three preset options, respectively.
・Aseptic production of nanoparticles is possible anywhere by using a sterilized syringes, tubing and microfluidic device. (No post-treatment mechanism such as ultrafiltration is provided.)
2-3. LiNAS-mini
・Basic model. Pump sold separately.
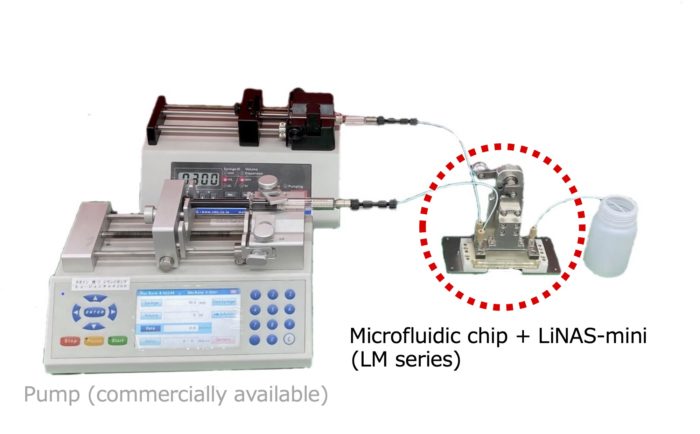
<Feature>
・The unique mechanism allows easy connection of the dedicated microfluidic chip and the pump.
LiMAP series (Sales scheduled for 2024 or later.)
・For GMP grade mass production of nanoparticles.
・Made-to-order machine.

