Case 1: Control of Liposome Particle Size by Total Flow Rate
– To investigate the particle size controllability characteristic of the iLiNP device, we measured the change in liposome average particle size as a function of flow rate variation.
Lipid: POPC
Device: iLiNP1.0S, iLiN1.0SW (wide channel)
Flow conditions: TFR=0.1-7.8mL/min, FRR=5
– After mixing POPC/ethanol solution and saline solution in the iLiNP device, the average particle size of liposomes generated was measured.
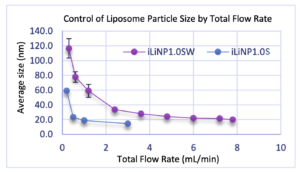
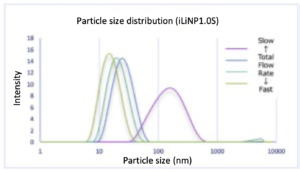
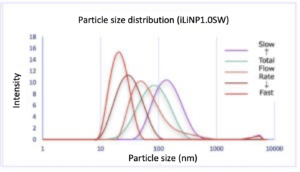
– Both devices were able to vary the average particle size significantly by changing the total flow rate.
Case 2: Control of poly(A)-LNP Particle Size by Total Flow Rate
– To investigate the particle size controllability characteristic of the iLiNP device, we measured the change in poly(A)-LNP average particle size as a function of flow rate variation.
Lipid: Ionizable lipid + helper lipids
Device: iLiNP-RoA (new model)
Flow conditions: TFR=0.3-20mL/min, FRR=3
– After mixing lipid/ethanol solution and poly(A)/acetate buffer solution in the iLiNPdevice, the average particle size of poly(A)-encapsulated lipid nanoparticles generated was measured.
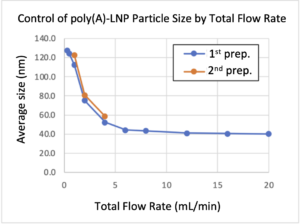
– Even in the case of nucleic acids-encapsulated LNPs, the average particle size could be varied significantly by changing the total flow rate.
– We have confirmed that there is no problem in the reproducibility of the production of LNPs with a particle diameter of 80-100 nm, which is a frequently requested range.
– It should be noted that the range of change in particle size varies greatly depending on the lipid used, the nucleic acid encapsulated, and the concentration of the lipid. For example, the particle size may not be smaller than expected in the case of payload with a large molecular weight.
Case 3: Manufacturing of mRNA-Encapsulated Lipid Nanoparticles and Evaluation of Effect of Remaining Ethanol on mRNA Delivery Activity
– Manufacturing of mRNA-encapsulated lipid nanoparticles by using the iLiNP device.
Lipids: ALC-0315 + helper lipids.
Payload: Fluc mRNA (5moU)
Device: LM-iLiNP001 equivalent (handmade device)
Flow conditions: TFR=0.5mL/min, FRR=3
Post-treatment: dialysis against PBS buffer
– After the dialysis, Ethanol (final conc: 9% or 18%) is added to the LNP suspension.
– The LNP suspensions (0.01 mg/mouse as mRNA dose) was inoculated by a single intramuscular injection into the thigh of one hind paw of ICR mice, and 1 day, 3 days, 1 week and 2 weeks later, the expression level of luciferase was quantified as luminescence.
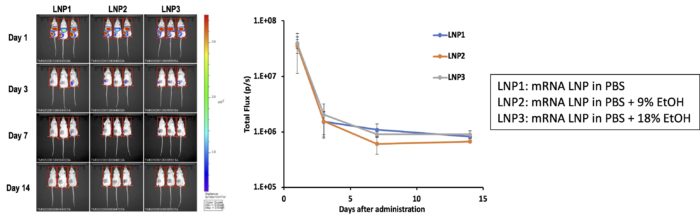
– The mRNA LNPs manufactured by the iLiNP have enough activity to deliver the mRNAs into the cells.
– The mRNA LNPs manufactured by the iLiNP are tough enough and keep delivery activity even in the presence of high concentration of Ethanol.
Case 4: Preparation of O/W Type Emulsion
– The iLiNP device can also be used to produce particles (droplets) other than lipid nanoparticles. As an example, an O/W type emulsion (oil emulsion) was prepared using the iLiNP device, in which oil and water are vigorously mixed in a channel in the presence of a surfactant to form micro-droplets.

Photo 1. O/W emulsions of baby oil (liquid paraffin) made under different flow conditions. Synthetic surfactant used. Oil concentration is about 18%. Creaming occurs in the conditions A and B because large droplets were generated under these conditions. On the other hand, relatively small and uniform droplets were generated in the conditions C, D, and E, and thus no creaming is observed.

Photo 2. Stereomicroscopic images of the samples. In the conditions A and B, coexistence of large droplets is observed, but in the conditions C, D, and E, coexistence of large droplets is suppressed and the particle size is homogenized.
– The iLiNP has an excellent ability to control the mixing state by adjusting the flow rate, so it is possible to obtain droplets of the targeted particle size in a single step. As a result, “creaming,” which tends to occur when large droplets coexist, is expected to be less likely to occur.
– The use of a palm-sized microfluidic device makes it possible to study emulsification conditions even with extremely small amounts of raw material liquid (~1mL). The small size of the device also makes on-site small-volume production of emulsions possible. If scaling up is required, the system can be flexibly adapted to meet demand by parallelizing and stacking the flow path chips.
Case 5: Preparation of PLGA Microspheres (Sustained Release Formulation)
– Micrometer-order particles can be prepared using the iLiNP device. As an example, microspheres made of PLGA (polylactic acid/glycolic acid copolymer), a biodegradable polymer, have been prepared. The microspheres can be used as a sustained release drug delivery system by encapsulating drugs.
– In the experiment, PLGA was dissolved in an organic solvent and mixed with an aqueous Camostat solution (model drug) to form a W/O emulsion, which was then suspended in a PVA solution to form a W/O/W emulsion. PLGA microspheres with an average particle size of approximately 0.5 μm and drug inclusion rate >99% were then obtained by removal of solvents.
– In each emulsification process, the iLiNP devices were used. Furthermore, by serializing iLiNP devices, W/O/W emulsions were successfully prepared continuously in one step from the raw material solution. By adjusting the pumping conditions, the particle size of microspheres can be further increased.

Case 6: Preparation of Emulsified Flavor
– As an example of application of emulsion preparation technology, we have made a prototype emulsified flavor.
– Emulsified flavor was obtained by mixing and emulsifying oil-soluble flavor (containing ethanol) and water in the presence of edible emulsifier, using the iLiNP (not-for-sale version) for emulsion production. Although the prepared sample contains about 25% ethanol, no increase in particle size or phase separation (creaming) was observed even after long-term storage at 4°C, and the particle size distribution remained unimodal. Samples in which ethanol is removed by dialysis also show similar stability.
– The high stability of the prototyped emulsified flavor is assumed to be due to the high particle size control characteristics of the iLiNP device.


